|
|
|
| |
|
> About | The DEPICT database A systematic review was conducted to create a database of relevant articles reporting the impact of clinical pharmacy services on the medication use process and patient health outcomes. The initial search focused on randomized controlled trials (RCTs) published up to November 30, 2014, in PubMed, Scopus, DOAJ, and SciELO. This search was supplemented by a manual review in the references of the included studies. Additionally, to complement the database, an overview of systematic reviews examining the impact of clinical pharmacy services was conducted. Systematic reviews published between 2000 and 2010 were searched in PubMed in December 2012. Only those that met items 4, 7, and 9 of the 2010 PRISMA statement checklist and included at least one RCT, were included. Finally, all RCTs from each systematic review that met the inclusion criteria were extracted. As a result, the DEPICT database comprised 569 articles and 488 studies (RCTs) from these searches. Starting in 2019, a new database was developed by conducting a systematic review of systematic reviews with meta-analyses (SRMAs), also known as an overview or umbrella review, to assess the impact of clinical pharmacy services. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the number CRD42024580944. Systematic searches (updated on Jan 1st, 2023) were conducted in PubMed, Scopus, and Web of Science without restrictions on timeframe or language. Additionally, manual searches were performed on the reference lists of the included studies. Systematic reviews with meta-analysis of interventional or observational primary studies comparing clinical services provided by pharmacists with those provided by other healthcare professionals or usual care were included. Usual care was defined as the routine care provided to patients in standard practice. Studies were excluded if they met any of the following criteria: a) articles written in non-Roman characters; b) systematic reviews without meta-analysis; c) outdated meta-analysis (only the most recent version was included to avoid duplicate results); d) interventions or services provided by a multidisciplinary team without distinguishing the pharmacist's role; and e) books, book chapters, dissertations, theses, and conference abstracts. All steps of the study selection process (title and abstract screening, and full text eligibility) were independently performed by two reviewers. A consensus meeting between the two researchers was held to resolve discrepancies. If any remained, a third researcher made the final decision. The flowchart of selection process is depicted below: 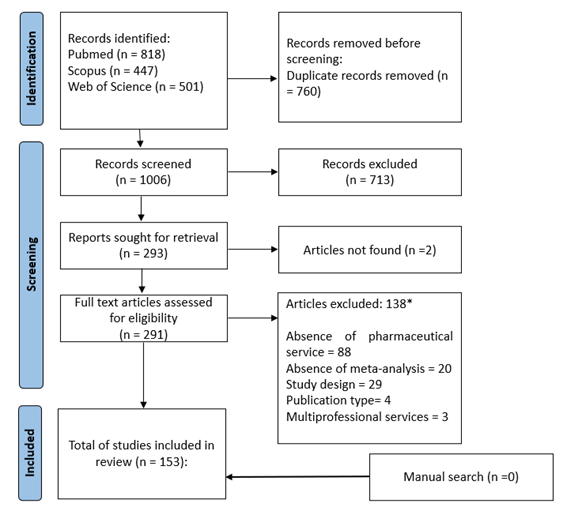 Thus, the current DEPICT database comprises 153 systematic reviews with meta-analysis. |
Institutions: | |
|
| |||


